How a multifaceted approach can help the industry improve efficiency and throughput
Despite being one of the largest contributors to global R&D spending, the pharma industry experiences some of the worst returns on its expenditures.
Long development times, process inefficiencies, regulatory challenges and uncertain outcomes all contribute to poor returns. This poor performance not only limits innovation, but also negatively impacts patients. Poor R&D performance can also lead to a lack of trust in the industry and the regulatory agencies that oversee it, which can have even broader implications for public health and safety.
Thus, the importance of improving R&D in pharma cannot be overstated. As the largest global net investor in R&D, the potential financial benefits of improving R&D in the pharma industry are enormous. Studies show that companies that are more efficient in innovation have higher market valuations, superior future operating performance and higher stock returns.
In order to address the issue of R&D performance in pharma, the industry needs to take a closer look at techniques that have proven effective in other industries — namely the use of lean development methods.
By adopting lean techniques and embracing innovation, the pharma industry can improve both top-line growth and bottom-line performance. Perhaps more importantly, the pharma industry has an opportunity to transform innovation and create a brighter future for patients, health care providers, and society as a whole.
...opportunity to transform innovation and create a brighter future...
Current state of R&D in pharma
According to a report by Deloitte, global R&D spending in the life sciences industry as a whole (pharma and medtech) exceeded $182 billion in 2020, making it one of the largest R&D spending sectors in the world.[1] Despite this significant investment, the industry has consistently realized poor returns on their innovation investment.
A report by McKinsey & Company revealed that the pharma industry has the lowest return on R&D investment compared to other sectors.[2] The report found that between 2010 and 2019, the top 12 pharma companies spent a total of $1.1 trillion on R&D and generated $1.2 trillion in revenue. This translates to an average return on investment (ROI) of only 1.8%, which is significantly lower than the average ROI of 9.6% for the technology industry and 6.5% for the consumer goods industry during the same period.
[1] "2020 Global life sciences outlook: The future awakens"
[2] "Biopharma productivity: The road ahead"
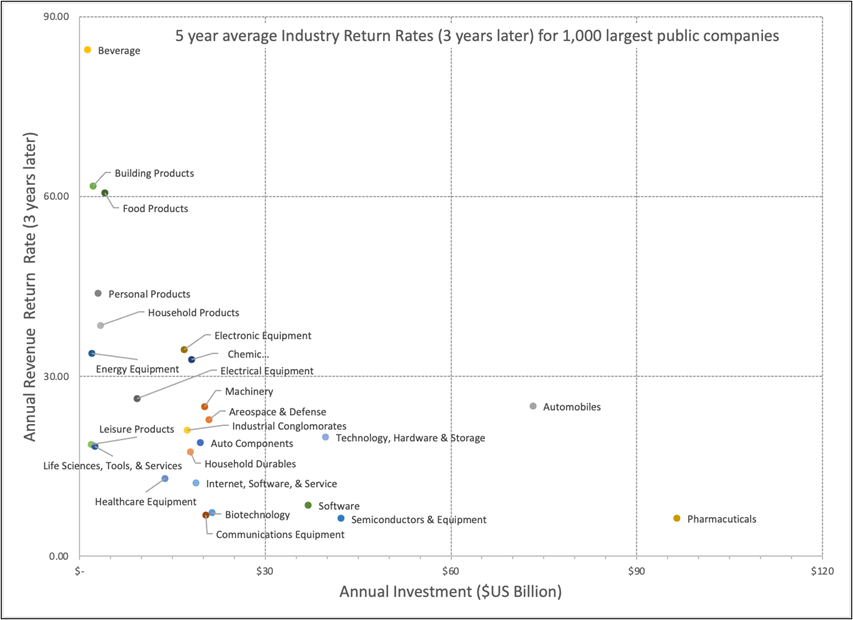
Source: Argo-EFESO research
These statistics highlight the significant challenge that the pharma industry faces in realizing returns on its investment in R&D. The low ROI makes it difficult for companies to attract funding for new R&D initiatives, and it also raises questions about the sustainability of the current R&D model.
Pharma companies face significant financial pressures due to the high cost of R&D, the long development time, and the uncertainty of success for new drug candidates. Moreover, the industry is subject to intense regulatory scrutiny, with strict requirements for safety and efficacy testing before drugs can be approved for use.
While it is true that regulatory requirements can be complex and time-consuming, it is important to recognize that other industries face similar challenges and still manage to achieve strong returns on innovation investment. Therefore, regulatory aspects cannot solely be used as a rationale for poor performance in the pharma industry.
Furthermore, studies have shown that a lack of innovation and inefficient R&D processes are often the root cause for poor financial performance in pharma companies, rather than regulatory constraints. For example, a McKinsey & Company report found that the top-performing pharma companies were able to effectively navigate the regulatory landscape while also maintaining a strong focus on innovation and efficiency.
Therefore, it is crucial for pharma companies to adopt new approaches and methodologies to improve their R&D performance and achieve better financial returns, while still meeting regulatory requirements.
Lean development as a solution
One potential solution is at the intersection of lean development methods with AI, which can help companies streamline their R&D processes and achieve greater efficiency and drive more effective innovation.
We suggest the companies explore three specific areas that are crucial to addressing the challenges faced by the pharma industry in realizing better returns on its R&D investments. These areas include:
- Leveraging lean development methods: Adopting lean development methods could significantly improve the efficiency and effectiveness of R&D in pharma, leading to more reliable outcomes and faster time to market.
- Embracing a culture of set-based experimentation: The ability to experiment and iterate quickly is critical to innovation, and pharma companies need to cultivate a culture of experimentation to achieve better outcomes.
- Strengthening collaboration and knowledge sharing: R&D in pharma is a highly collaborative process that often involves teams across multiple locations and organizations. Improving collaboration can create reusable knowledge and knowledge sharing can lead to better outcomes.
The traditional approach to drug development in the pharma industry has been characterized by a linear, sequential process that can be slow and inefficient. This approach can result in a significant amount of time and money being invested in compounds that ultimately fail to make it to market.
Instead, we propose a multifaceted approach that focuses on three key areas:
- Reducing time to market: One of the primary ways to improve the return on investment in R&D is to reduce the time it takes to bring a drug to market. This can be achieved through the use of more agile and flexible development methodologies, such as lean development and agile methodology, that prioritize speed and efficiency.
- Increasing throughput: Another key area of focus is increasing the throughput of the drug development process. This can be achieved through the use of automation and other technologies that can help to streamline the drug development process, reducing the time and cost required to bring a new drug to market.
- Improving uncertainties associated with the development stream: Finally, we propose improving the uncertainties associated with the development stream, including improving the predictability of drug development outcomes, reducing the likelihood of late-stage failures, and increasing the success rate of clinical trials. This can be achieved through the use of new technologies such as machine learning and artificial intelligence, which can help to improve the predictability of drug development outcomes and identify potential issues earlier in the development process.
By focusing on these key areas, we believe that it is possible to significantly improve the return on investment in R&D in the pharma industry, while also bringing much-needed therapies to patients more quickly and efficiently.
A call to action
With the growing need for innovative therapies to address unmet medical needs, there is an urgent need for the pharma industry to improve its R&D processes.
While there are multiple factors contributing to the poor returns on pharma R&D, there are also promising solutions available, including lean development methods. Reducing time to market, increasing throughput, and improving uncertainties associated with the development stream are crucial areas of focus.
It is imperative for stakeholders in the industry to prioritize and invest in a multifaceted approach to R&D improvement. This includes adopting more efficient and effective development methods, leveraging data and technology to enhance decision-making, improving collaboration between industry partners and addressing regulatory challenges.
Therefore, we call on leaders in the pharma industry to take action and invest in R&D improvement. This requires a commitment to change, a willingness to embrace new approaches, and a recognition of the long-term benefits that will result from such investment. By doing so, we can help ensure the future success and sustainability of the pharma industry.
Questions;
Do you agree with how these methodologies are characterized?
What methodology does your organization of visual communitcation?
Join the conversation at our LinkIn group!
- References
- (2018). Measuring the return from pharmaceutical innovation 2018. Retrieved from https://www2.deloitte.com/content/dam/Deloitte/uk/Documents/life-sciences-health-care/deloitte-uk-measuring-the-return-from-pharmaceutical-innovation-2018.pdf
- McKinsey & Company. (2018). Pharma’s next challenge: Delivering on product launch excellence. Retrieved from https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/pharmas-next-challenge-delivering-on-product-launch-excellence
- Biotechnology Innovation Organization. (2021). The biopharmaceutical pipeline: Evolving science, hope for patients. Retrieved from https://www.bio.org/sites/default/files/2021-03/BIO%20Industry%20Analysis%202020%20FINAL.pdf
- Competitiveness Index: Where America Stands Council on Competitiveness. (2007). Retrieved from http://www.compete.org/publications/detail/137/competitiveness-index-where-america-stands-2007-edition
- Morbey, J., & Reithner, M. (1990). The relationship between R&D intensity and growth in sales in Canadian and US manufacturing. Research Policy, 19(5), 425-438.
- Hirshleifer, D., Hsu, P. H., & Li, D. (2013). Innovative efficiency and stock returns. Journal of Financial Economics, 107(3), 632-654.
- Kim, C., & Mauborgne, R. (2015). Blue ocean strategy, expanded edition: How to create uncontested market space and make the competition irrelevant. Harvard Business Review Press.
- Clark, J. W., & Hodgson, D. (2018). Designing agile organizations: A new approach to designing businesses for disruption and growth. John Wiley & Sons.
- Radziwill, N. M. (2018). The benefits of lean Six Sigma in healthcare. Journal of Healthcare Engineering, 2018, 8373649.
- Porter, M. E., & Lee, T. H. (2013). The strategy that will fix health care. Harvard Business Review, 91(10), 50-70.
- Adams, C. P., & Brantner, V. V. (2010). Estimating the cost of new drug development: is it really $802 million?. Health affairs, 29(1), 87-95.
- Deloitte. (2020). The future awakens: Life sciences and health care predictions 2021. Deloitte Insights.
- European Commission, JRC/DG RTD. (2021). The 2021 EU Industrial R&D Investment Scoreboard.
- Hirshleifer, D., Hsu, P. H., & Li, D. (2013). Innovative efficiency and stock returns. Journal of Financial Economics, 107(3), 632-654.
- James, J. S. (2018). Slow Progress on Fighting Antibiotic Resistance in the US. Journal of the International Association of Providers of AIDS Care (JIAPAC), 17, 2325958218787330.
- Lerner, J. (2019). The failure of drug development and regulatory reform. Clinical Pharmacology & Therapeutics, 106(1), 37-46.
- Morbey, M. L., & Reithner, R. (1990). R&D intensity and corporate financial policy: Some international evidence. Journal of Business Finance & Accounting, 17(1), 1-17.
- Oosterwal, D. (2010). The lean machine: How Harley-Davidson drove top-line growth and profitability with revolutionary lean product development. AMACOM Div American Mgmt Assn.
- Paul, S. M., Mytelka, D. S., Dunwiddie, C. T., Persinger, C. C., Munos, B. H., Lindborg, S. R., & Schacht, A. L. (2010). How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nature Reviews Drug Discovery, 9(3), 203-214.
- PhRMA. (2020). Biopharmaceutical Research & Development: The Process Behind New Medicines.

Dantar Oosterwal
Partner & Senior Vice President, ARGO-EFESO
Dantar Oosterwal has a passion for Innovation and an enthusiasm for the improvement of Business Systems. At the intersection of these two interests, Dantar is a leader in the application of lean methods in new product development creating dramatic improvements in effectiveness and efficiency. Besides the award winning book, ‘The Lean Machine’, Dantar has also co-authored together with Durward Sobek the publication of Allan Ward’s manuscript, ‘Visible Knowledge for Flawless Design’.You can learn more at http://theleanmachine.org/ Or you can learn about Dantar’s latest initiative at DevelopLean.com Dantar holds a BS degree in Mechanical Engineering from The University of Michigan and a Masters degree in Management from the Sloan School of Management at MIT.




